Our recent publication in Nature Microbiology presents findings elucidating the complex dynamics of gut microbial communities, with a focus on the role of plasmids in shaping these interactions.
Below is a summary of our main findings:

In the lively world of gut microbes, a fascinating tale of microbial warfare and peace unfolds—a story in which plasmids emerge as key players. Plasmids, often likened to “mini-chromosomes,” are genetic backpacks bacteria carry alongside their primary genome, offering additional genes that confer various benefits, from antibiotic resistance to toxin production.
Despite their significance, the precise role of plasmids in gut microbial communities has remained largely unexplored.
Our team embarked on a journey to unravel this mystery: to understand how plasmids shape microbial interactions within the gut. Our quest led us to a discovery of a plasmid carrying the 1,3-PD gene, widespread across gut ecosystems. Intrigued by its prevalence, we delved into its function and its impact on microbial communities. Through a blend of cutting-edge analyses and traditional investigative methods, we uncovered the plasmid’s remarkable defense mechanism. It equips bacteria with a shield against reuterin, a potent toxin produced by certain gut microbes. But here’s where the story takes an unexpected turn: by resisting this toxin, bacteria form alliances, fostering mutually beneficial relationships instead of engaging in relentless warfare.
Picture this: former rivals joining forces, sharing resources, and thriving together. That’s the essence of microbial diplomacy within our guts. Bacteria armed with plasmid-encoded defenses cozy up to toxin producers like L. reuteri, engaging in a microbial exchange program where both parties reap the rewards.
Our discovery isn’t just intriguing; it has profound implications for gut health. The prevalence of these plasmids suggests that toxin resistance plays a pivotal role in shaping gut ecosystems, potentially extending its influence to humans.
So, what does this mean for us? It challenges the notion of bacteria as solitary warriors, instead portraying them as shrewd diplomats, negotiating peaceful coexistence through genetic weaponry.
As we continue to unravel the intricacies of microbial diplomacy, we gain valuable insights into promoting gut health and combating diseases.
Understanding this phenomenon offers a pathway to deciphering the mysteries of gut health and disease prevention. So, the next time you ponder your gut, remember—it’s not just a battleground but a bustling arena of microbial diplomacy, where plasmids hold the keys to peace.
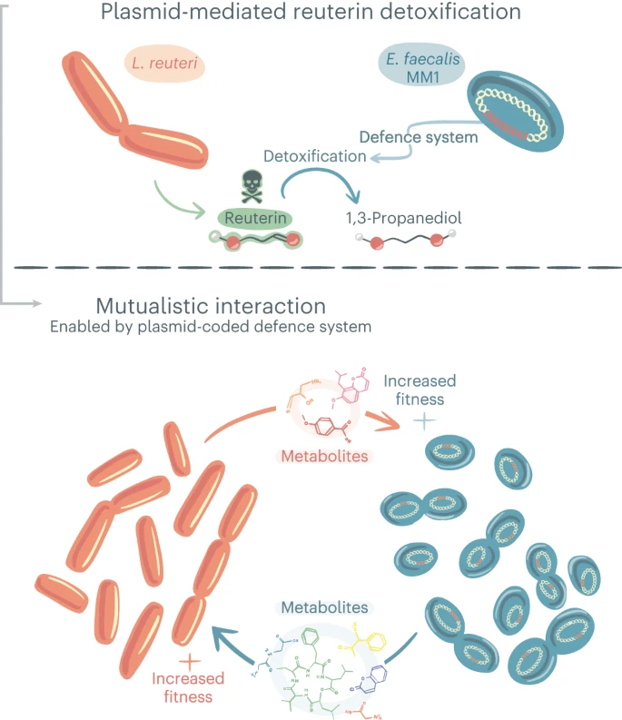
The antibacterial toxin, reuterin, produced by L. reuteri (in orange) is detoxified by the plasmid-encoded defence gene (in red) of E. faecalis (in blue), thus allowing E. faecalis survival and use of L. reuteri-produced metabolites to increase its fitness. The beneficial effect becomes mutual when the metabolites produced by E. faecalis are consumed by L. reuteri, which in turn also increases the latter’s fitness.