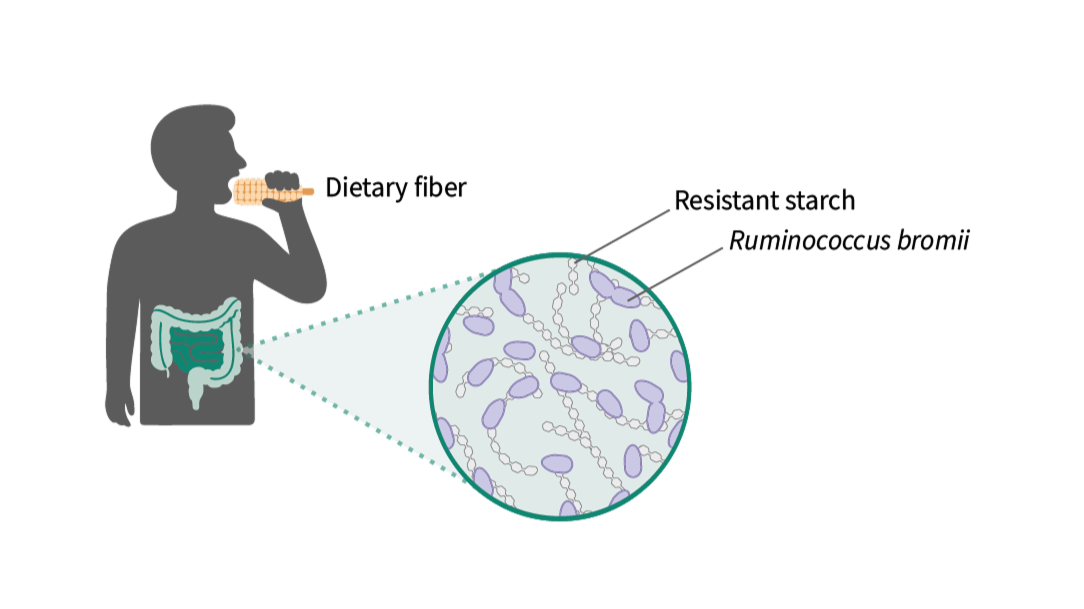
We all know dietary fiber is good for us, but have you ever wondered why? Dietary fibers are special because they resist digestion in our stomach and small intestine, reaching the colon intact. There, beneficial gut bacteria ferment these fibers into short-chain fatty acids (SCFAs)—powerful molecules that reduce inflammation, regulate blood sugar, and protect gut health.
A particularly important type of dietary fiber is resistant starch (RS). Resistant starch is abundant in everyday foods like cooked and cooled potatoes, green bananas, whole grains, legumes, rice, and pasta. However, humans can’t digest resistant starch on their own—we depend entirely on our gut microbiome to unlock its benefits.
Ruminococcus bromii, a remarkable gut bacterium that specializes in breaking down resistant starch. In fact, R. bromii is so efficient at this task that it’s considered a “keystone species,” meaning it doesn’t just nourish itself; it also produces nutrients that help feed many other beneficial bacteria. By kickstarting the fermentation of resistant starch, R. bromii helps maintain a healthy, balanced gut ecosystem.
But how exactly does R. bromii perform this crucial role? The answer lies in a fascinating structure known as the amylosome—a dense, enzyme-packed complex located on the bacterium’s cell surface. Until now, how this sophisticated molecular machine operates and adapts to our diet was largely unknown.
In our new preprint, “Spatial constraints drive amylosome-mediated resistant starch degradation by Ruminococcus bromii in the human colon”, we dove deep into this microscopic world to understand how R. bromii uses its amylosome to unlock resistant starch. Combining advanced techniques—including cryo-electron tomography (cryo-ET), proteomics, structural biology, and biochemical assays—we were able to visualize, for the very first time, how the amylosome assembles and functions at a nanoscale resolution.
An integrative model synthesizing our multi-layered data illustrates how the amylosome is spatially organized and dynamically regulated during starch degradation by R. bromii
We discovered several exciting things:
- Visualization of the Amylosome: For the first time, we captured images of the amylosome in action, revealing it as a stable, enzyme-packed layer tightly bound to the bacterial cell wall, reaching out towards starch granules.
- Dynamic Protein Composition: The bacterium adjusts the composition of its amylosome depending on the available diet. When resistant starch is abundant, R. bromii dramatically increases production of certain key enzymes within the complex.
- Enzyme Synergy and Complementarity: We uncovered that two enzymes, in particular, team up effectively. Individually, these enzymes break down starch moderately well, but when positioned close together within the amylosome, their combined activity dramatically enhances starch degradation.
- Two Layers of Regulation: The bacterium finely controls its starch-degrading capability by carefully placing enzymes in precise positions within the amylosome (spatial constraints) and regulating how much of each enzyme it produces (expression tuning).
In simple terms, R. bromii carefully arranges and balances its starch-degrading enzymes like players on a sports team, ensuring maximum efficiency and effectiveness.
Why does this matter? Understanding how R. bromii adapts to our diet could help us design better nutritional guidelines and probiotic therapies. By knowing how to support beneficial bacteria that efficiently unlock dietary fiber, we could potentially improve gut health, metabolism, and overall wellbeing.
Check out our preprint here: [bioRxiv link]