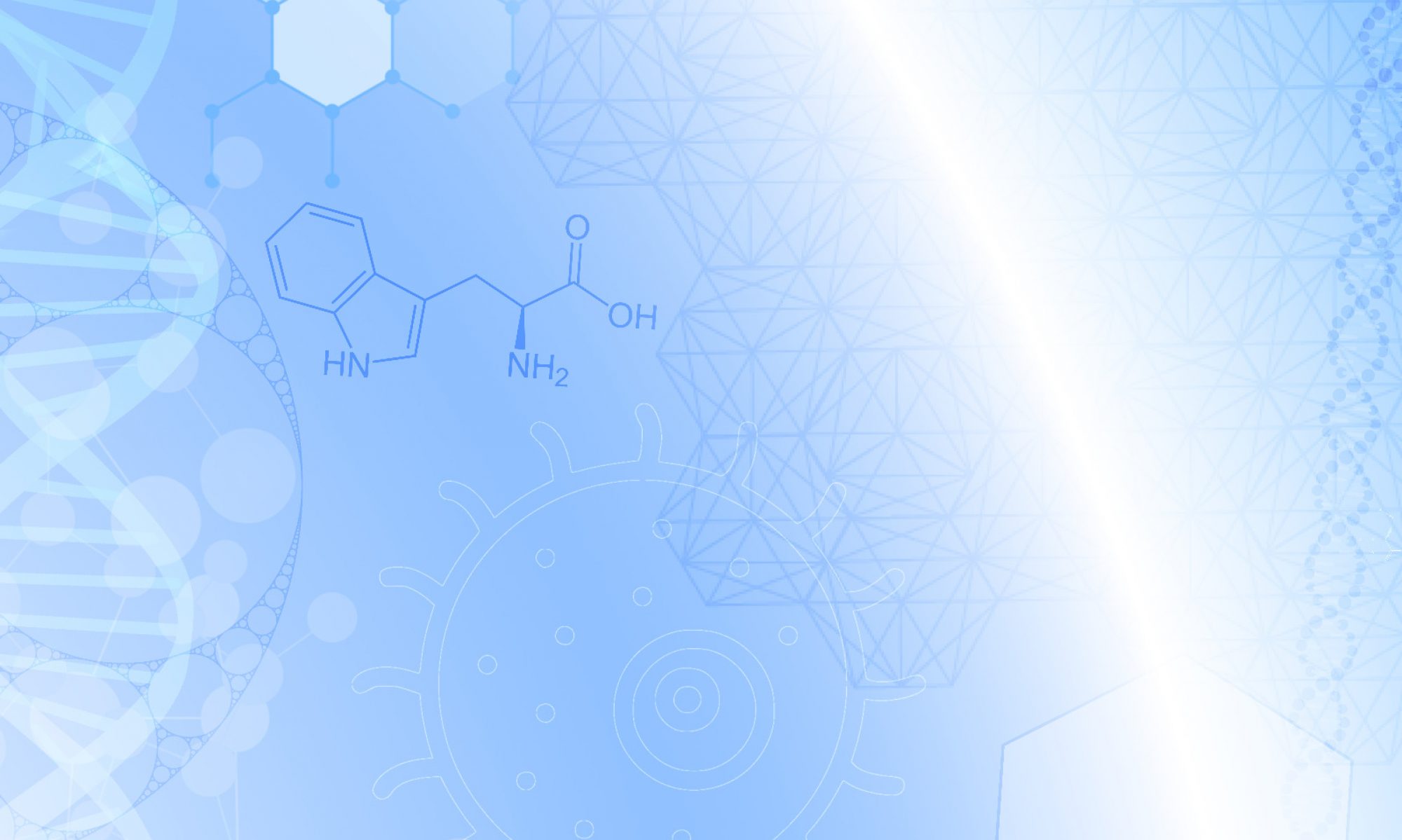
Our lab focuses on two complementary model systems that illustrate how proteins integrate environmental signals to make functional decisions:
AAA+ ATPases
We study Mpa, the proteasomal ATPase of Mycobacteria. Mpa is a highly dynamic molecular machine that orchestrates substrate identification, unfolding, and translocation into the 20S proteasome. This system exemplifies how proteins integrate mechanical, structural, and nucleotide-derived signals to regulate substrate processing—a key decision in proteostasis.
Mpa is essential for the survival and virulence of M. tuberculosis, making it a promising target for novel antibacterial strategies.
Iron Homeostasis
We investigate the iron-storage protein ferritin and its regulation by the adaptor protein NCOA4 and the E3 ubiquitin ligase HERC2, which together form the Ferritin–NCOA4–HERC2 iron regulatory circuit. This network controls ferritin degradation in response to fluctuations in intracellular iron levels and redox state, integrating metabolic, oxidative, and signaling cues to regulate iron availability.
Dysregulation of this circuit is implicated in conditions such as neurodegeneration, anemia, and cancer, underscoring its importance in both cellular function and human health.