Major Depressive Disorder (MDD), commonly known as “clinical depression”, is a brain disease with high personal and social costs, and is one of the most prevalent psychiatric disorder today. The neurophysiological mechanisms underlying MDD are still obscure, however it appears that specific alterations in the Reward Pathway may contribute its development.
Animal studies
A genetic model for MDD
Genetic determinants appear to significantly contribute to the development of MDD, however the specific genes involved have not yet been identified. We have developed a unique rat model for MDD, based on a multifactorial selective breeding for depression-like behaviors, which can be studied to understand the neurophysiological and genetic correlates of MDD-like behaviors. After several generations of such selective breeding, our depressive-rat-line (DRL) rats show intrinsic depressive-like behaviors. We then study the Reward Pathway in the brain of these rats using neurochemical analyses and electrophysiological recordings, and try to manipulate these circuitry and normalize the rats’ behavior using pharmacological treatments or electrical brain stimulations.
Molecular investigations
We focus on Brain Derived Neurotrophic Factor (BDNF) as a factor of brain plasticity changes involved with the expression of depression-like behaviors in rats. We use molecular tools (including lentiviral vector-induced BDNF knockdown) to manipulate BDNF expression in the rat’s brain. We use neurochemical analysis methods to study how BDNF expression changes in depression-like states, and how brain stimulation affects these changes in accordance with behavior.
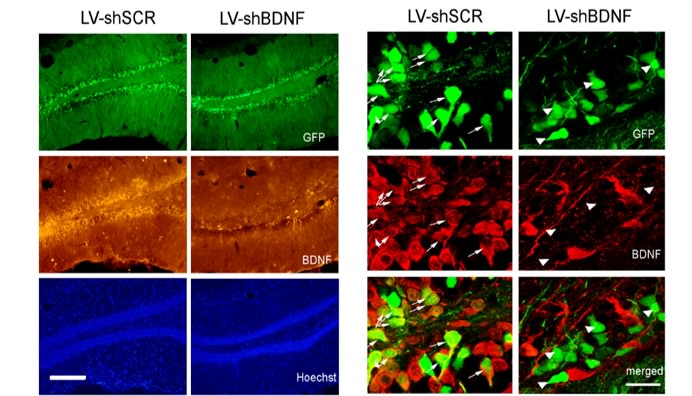
Knocking-down BDNF in the rat’s brain. Hippocampal slices from control (left) and BDNF-knockdown (BDNF-KD, right) rats, produced with a lentiviral vector microinjection. Left: micrographs showing the infection spread based on the GFP expression (top), BDNF (middle), and cell nuclei (bottom). Right: higher magnification of an infected hippocampal region, showing GFP expression as an infection marker (top), BDNF protein staining (middle) and a superimposition (bottom) highlighting cells expressing both GFP and BDNF. Arrows highlight that while the control brain shows BDNF expression within infected (GFP) cells, none of the infected cells in the BDNF-KD brain shows BDNF expression. Adapted from Taliaz et al. 2010.
Attentional alteration in depression and following prefrontal cortex stimulation as a treatment for depression
Transcranial magnetic stimulation (TMS) can modulate cortical excitability and plasticity in humans and rodents. The increase of noninvasive electrical brain stimulation research in humans, both for experimental and clinical purposes, has yielded an increased need for basic, mechanistic, safety studies in animals, and the DRL is one such model.
Formerly, we have demonstrated that electric stimulation of the prelimbic cortex (PL) in the DRL model was associated with reduction of depressive-like behaviors. Of note is that treatment was done using TMS-like parameters of stimulation, and that together with the infralimbic cortex (IL), the PL is considered to be homologous to the dorsolateral prefrontal cortex (DLPFC) of humans – a target for TMS treatments for both depression and attention deficit hyperactivity disorder (ADHD).
The current study utilizes the DRL model in order to research attentional deficits related to depression. Difficulty concentrating, which has been related to reduced attentional control, is considered to be a characteristic symptom of depressive episodes. Moreover, it was argued that attentional factors are of crucial importance in understanding increasing vulnerability for depression, as they play an important role in the etiology and maintenance of depression and contribute to relapse following remission.
We are examining the effects of PL and IL stimulation and examine the behavioral and electrophysiological correlates of attentional factors in DRL rats, compared to MRL and wild-type (WT) rats. More specifically, we are comparing reaction to attentional grabbing stimuli, before and after exposure to TMS-like treatment (using implanted electrodes and electrophysiological recording) in the five-choice serial-reaction time task (5-CSRTT).
Human studies
Pharmacological treatments available today for MDD suffer numerous caveats, including low efficacy and adverse side effects. In fact, the best treatment available today is electroconvulsive shock therapy (ECT), which can ameliorate MDD in many drug-resistant patients. However, this convulsive treatment possess numerous side effects and is therefore used only in extreme cases. Similar to ECT, we study the possible treatment of MDD using electrical stimulation of deep brain regions; however, unlike ECT, we use the deep-TMS coil to induce focal subconvulsive electrical currents in chosen brain regions. A recently concluded clinical trial has shown dramatic improvement of MDD patients following this safe and non-invasive technique.

The deep-TMS system used to treat Major Depressive Disorder
Personalized treatment of depression – establishing EEG-based prognostic biomarkers for medial and lateral deep transcranial magnetic stimulation (dTMS)
The aim of this study is to establish EEG based biomarkers for personalized treatment of depression based on two different deep TMS targets: the standard dTMS treatment of lateral prefrontal cortex (L-PFC) is compared to medial PFC (M-PFC) dTMS treatment. The latter is based on initial evidence where dTMS of the M-PFC was found to be an effective treatment for MDD patients who failed L-PFC dTMS, with 56% remission. We are searching for biomarkers embedded in the EEG activity recorded during different conditions, including baseline resting state and response to TMS evoked potentials (TEPs); but mostly we are interested in the low gamma to alpha activity ratio during the first treatment, which was already established in our previous studies as a high level biomarker predicting dTMS outcome. Identifications of such biomarkers will allow future assignment of patients for treatment using the most suited coil, and will increase the total effectiveness of dTMS treatment.
