Drug addiction, also known as substance dependence, is a compulsive use of a substance despite its adverse consequences (e.g., monetary costs, health hazards etc.). When the drug use is discontinued (abstinence), withdrawal symptoms appear and promote relapse to drug use. Other factors promoting relapse are stress, re-exposure to the drug itself (“priming”) and re-exposure to environmental cues that were previously associated with the drug (i.e., a cigarette pack, a rolled-up dollar bill, etc.) A common circuitry involved in the development of addiction to different drugs, from nicotine to heroin, is the brain’s Reward Pathway. This pathway comprises several brain structures, most prominently the ventral tegmental area (VTA), the nucleus accumbens (NAc) and the prefrontal cortex (PFC), together known as the mesocorticolimbic pathway. This pathway naturally serves to promote learning of reinforced (rewarded) behaviors, and is largely mediated by the neurotransmitter dopamine. Repeated use of addictive drugs affects neuroplasticity in this pathway, resulting in the reinforcement of drug-related behaviors (= addiction). Our lab studies the changes occurring in the Reward Pathway due to the different stages of addiction, including the development of the drug-seeking behaviors, the emergence of withdrawal symptoms during abstinence from drug use, and the causes of relapse to drug use. We study these components on both animal models and human subjects.
Animal studies
Behavioral paradigms
We have developed a naturalistic model of drug addiction and relapse in rats. This “Conflict Model” considers that abstinence from drug use in addicts is the result of an ongoing conflict between the positive and negative effects of the drug, i.e., a cognitive “battle” between the euphoric effects and the negative consequences of drug-use. In the Conflict Model we allow rats to choose whether or not to surpass an electric barrier and receive a foot-shock in order to self-administer a dose of the drug. Most rats will refrain from drug self-administration when the shock intensity is too high, and will thereby self-abstain from the drug. We then decrease the shock intensity and test whether the rats will relapse back to drug self-administration when presenting them with drug-associated cues (i.e., cue-induced relapse).


The conflict model for cue-induced relapse. Top: cocaine self-administration chamber with an electric barrier. Bottom: description of the conflict model procedure.
Electrophysiological recordings and electrical stimulation
We use electrophysiological recordings to chronically monitor neuronal activity in designated brain regions during the different stages of drug addiction. We focus on the PFC and how electrical activity in this region is altered in these different stages. We record both low-frequency (LFP) and high-frequency (spiking) activity from behaving rats in the Conflict Model. We also apply electrical stimulation to modulate neuronal activity.

Electrophysiological recording setup. Positioning of a Linear Microelectrode Array (LMA) in the rat’s prefrontal cortex. The array allows both stimulating and recording of spikes and LFPs.
Neurochemical analyses
We focus on markers of neuroplasticity such as Glutamate receptor (GluR) and Brain Derived Neurotrophic Factor (BDNF), which are involved with addiction-related long term changes in brain plasticity. BDNF abnormalities have been implicated in several psychiatric disorders, including in addiction. We use sensitive neurochemical detection tools (e.g., ELISA) to study how GluR1 and BDNF changes coincide with the development of addiction and relapse to drug use, and how brain stimulation alters their expression and change addiction-related behavioral parameters.
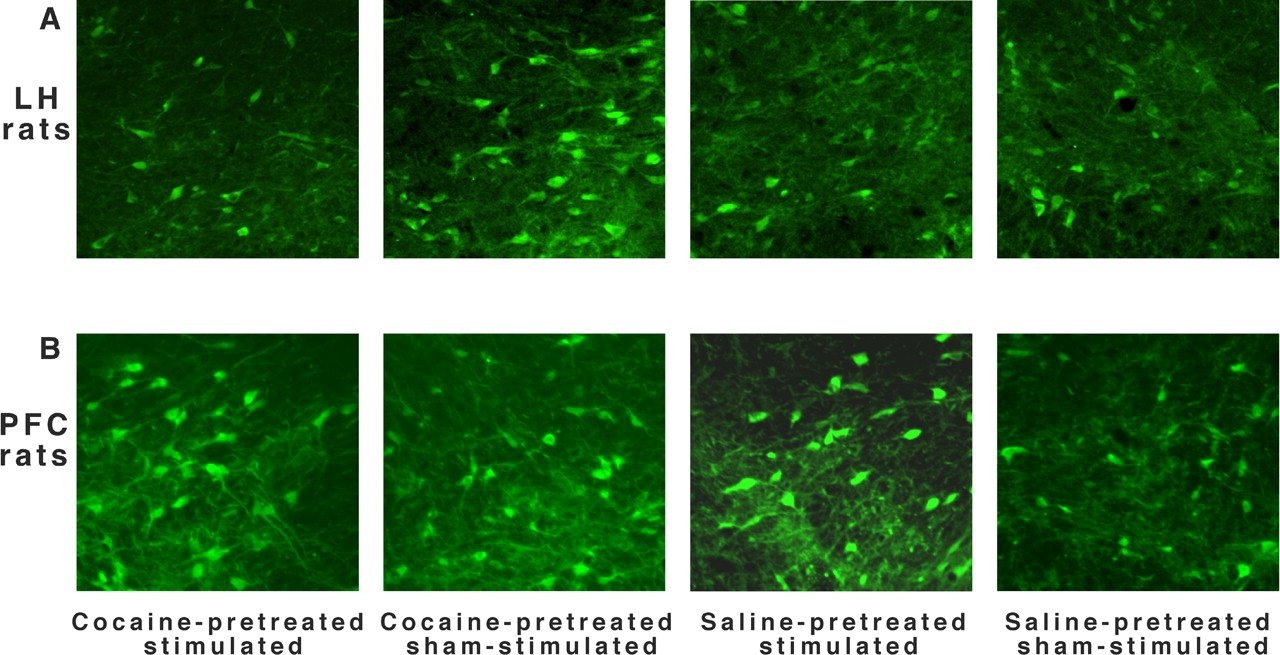
The effect of repeated cocaine and ICES on GluR1 in the anterior VTA. Representative immunohistochemical images of the anterior VTA from LH (A) and PFC (B) rats (Levy et al, 2007; JNS)
Human studies
We use deep transcranial magnetic stimulation (dTMS) to study and possibly treat addiction-related behaviors in humans addicted to nicotine, cocaine, heroin, or food. We stimulate deep brain regions (the PFC, cingulate cortex and/or insula) using novel specialized deep-TMS coils (specific version of the H-coil that we have developed), and combine this with EEG measures. We collaborate with several insitutes, in Israel and worldwide.
Exploration of the potential role of anterior cingulate cortex (ACC) dTMS in relapse to alcohol use
Alcohol use disorder (AUD) is a highly prevalent disorder in western countries, with high relapse rate and limited treatment options; partly due to ill-understanding of the neurocircuits involved. One of the hubs that was highlight in that regard is the anterior cingulate cortex (ACC), which is hypoactive in AUD and plays a central role in the behavioral symptoms, reduced inhibitory control, altered resting state connectivity and relapse rate. Here, we modulate ACC activity by deep transcranial magnetic stimulation (dTMS) in the attempt to reduce craving levels and relapse rates in short-term abstinent AUD subjects. We use fMRI and EEG to examine the response of the relevant neuronal network to treatment, and its correlation to clinical improvement.
Details for potential candidates can be found here

Electric field distributions of the TMS coil used in the study. The colored maps, overlaid on MRI images, describe the absolute magnitude of the electric field as measured in a phantom model of the human head. In the color scale, red indicates a field magnitude above neural threshold, while white and yellow indicate field magnitude below neural threshold.
Addictive eating in morbid obesity: behavioral and electrophysiological characteristics
Obesity is a growing public health problem with staggering economic and social implications. The incentive sensitization theory to obesity suggests that a neurocognitive malady is at the center of this condition, where obese individuals are over-sensitized to external food cues, thereby occasionally express food addiction (FA). FA can be measured with the Yale Food Addiction Scale (YFAS) and may indicate a distinguish obesity phenotype. According to the motivation-direction model, greater dominance of the left Pre-Frontal Cortex (PFC Left asymmetry) is linked with approach motivation and cue reactivity, while greater dominance of the right PFC (right asymmetry) is linked with avoidance motivation and inhibitory control. Thus far, the association between left asymmetry and addictive eating in obesity has not yet been explored, but according to the characteristics of FA (eg., food cue reactivity and impaired inhibitory control) it may be expressed in obese. Furthermore, shifting brain asymmetry to the right may help decrease cue-reactivity in response to food, thereby reducing symptoms of FA. A shift in brain asymmetry may be achieved via pairing the neurostimulation of two brain regions in a coordinated fashion.This is based on Hebb’s rule of plasticity, and can be achieved via dTMS in a protocol termed Paired Associative Stimulation (PAS), which can target the connectivity between the two brain regions.
